Abstract
Background
Endovenous stenting is a method to overcome venous outflow obstruction in the treatment of patients with acute iliofemoral deep vein thrombosis (DVT) or in those with symptomatic post-thrombotic syndrome (PTS). These treatments allow maximal luminal expansion of the caval and/or iliac venous segment leading to a reduction in venous hypertension. While there is growing enthusiasm for their use, particularly as conventional treatments usually with anticoagulation are not always effective there is however little clinical data regarding efficacy in improving clinical outcome.
Objectives
To determine the outcomes in patients following placement of venous stents for treatment of obstruction in acute venous thrombotic and post-thrombotic syndrome.
Methods
Consecutive patients with a history of DVT in whom a venous stent was inserted at two UK specialist tertiary referral centres between 2012-2015 were included. 'Acute' patients consisted of those with a fresh symptomatic iliofemoral DVT; chronic patients were those with recalcitrant PTS unresponsive to conventional therapy. Patients planned for intervention were discussed by a multidisciplinary team made up from Haematology, Interventional Radiology and Vascular Surgery.
Stents were placed under general anaesthesia. Venous access was obtained using ultrasound guidance. Intravascular ultrasound (IVUS) was used to size the stent and to ensure it was fully expanded. Endophlebectomy with arterio-venous fistula formation was carried out in selected patients. All patients were given therapeutic dose low molecular weight heparin (LMWH) post procedure before transition to oral anticoagulation.
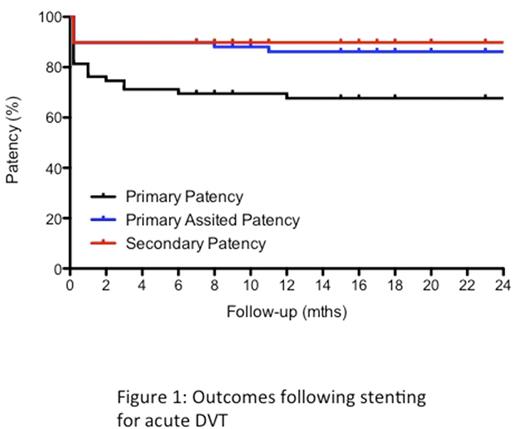
Ultrasound surveillance commenced the day after the procedure and at 2 weeks, 6 weeks, 3 months, 6 months, 1 year and annually thereafter. Clinical follow-up was at 6 weeks, 6 monthly thereafter. Primary patency was defined as a patent stent with <50% diameter reduction. Primary-assisted patency was defined as a stent that had not occluded, but had required a re-intervention to maintain patency, based on imaging findings and/or symptoms. Secondary patency included stents that had blocked and were successfully re-opened. Ulcer healing or changes in Villalta Score were used as measures of clinical outcome following intervention.
Results
379 venous stents were placed in 148 patients. The median age of patients was 42yrs (range: 18-81yrs), and 86 (58%) patients were female. Symptoms in the left leg were most common (116 patients, 78%).
There were 60 (41%) patients who had a venous stent placed to treat an underlying stenosis following catheter directed thrombolysis of an acute iliofemoral DVT. 88 (58%) patients had stent placement to treat a post-thrombotic obstruction considered pathological for PTS.
Primary, primary-assisted and secondary patency was 67%, 85% and 88% respectively at one-year in the acute DVT group (figure 1) and 64%, 86% and 86% respectively for the PTS group (figure 2). The median Villata score was 0 (range 0-14), 12 months after stenting, though new ulceration was noted in three patients in the acute DVT group. Median Villalta scores in patients without ulceration decreased from 15 (range 6-23) to 5 (range 1-22) one-year following the procedure (P<0.0001) in the PTS group with the greatest improvement in Villalta score in patients with a patent stent (P<0.0001). Ulcer healing occurred in 6 of 13 patients during follow-up. Four patients with severe pre-operative PTS developed an ulcer six-months following intervention.
There was one death from cancer during follow-up, but no stent related mortality or major bleeding complications.
Conclusions
This is the largest reported series of outcomes of venous stents placed in patients with acute iliofemoral venous thrombosis or severe post-thrombotic syndrome. Patients had a minimum follow-up of one year Venous stenting offer potential for the treatment of patients with obstruction related to thrombotic venous disease with good clinical outcomes at one year. Further study on patient selection, outcomes and optimal anticoagulation is required.
Breen: Veniti: Speakers Bureau. Cohen: Bayer Healthcare: Employment, Speakers Bureau. Uprichard: Bayer Healthcare: Speakers Bureau. Black: Veniti: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal